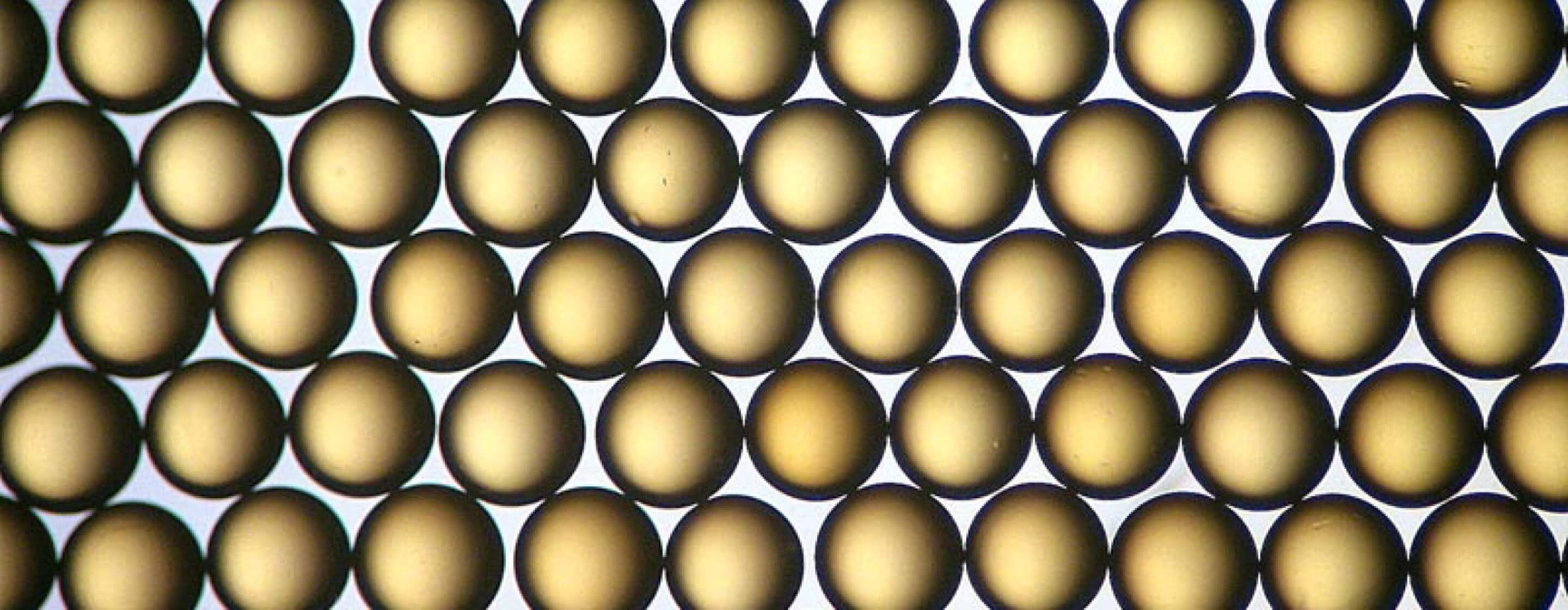
Effective ion exchange for challenging water-treatment needs
Ion exchange (IX) is the reversible interchange of ions between a solid and a liquid such as water. Ion exchange resins remove harmful contaminants from liquids, replacing them with beneficial, desired ions.
Ion exchange is used in water treatment, including water softening, industrial demineralization, condensate polishing, ultrapure water production, and wastewater treatment. It can also provide a method of separation in many nonwater processes, such as desiccation and chromatographic separation. It has special utility in chemical synthesis, manufacturing, food processing, mining, power generation, agriculture, and a variety of applications and other industries.
For more than 80 years, DuPont Water Solutions has developed one of the deepest ion exchange resin portfolios in the industry, working closely with customers like you to meet evolving separation and purification needs.
A closer look
Separating soluble substances by interchanging ions
Whereas solids can be separated from liquid media by means of filtration, soluble substances require different means. Ion exchange is the reversible interchange of ions between a solution with soluble ionized substances and a solid (the ion exchange material, such as a cation resin), in which there is no permanent change in the structure of the solid.
Fundamentally, the ion exchange process is this: A solution passes through or over ion exchange resins, which contain mobile or exchangeable ions. The solute ions that have a stronger affinity to the resins than the ions on the resins are removed from the solution and are replaced by the exchangeable ions on the resins. In water processing, ion exchange typically removes hardness in softening applications and all dissolved ions in demineralization. Ion exchange is also used to remove nitrate, chromate, arsenic, and other specific contaminants from drinking water.
Ion exchange processes can be conducted in continuous or batch mode, with most water treatment IX processes run in a continuous mode. Continuous ion exchange processes take place in a column or vessel containing a deep bed of ion exchange resin beads; water typically flows down through the resin bed. When resin capacity becomes exhausted, the resins are regenerated.
Structure and traits
Approximately 85 percent of ion exchange resins in the market are composed of a polystyrene matrix. Another 10 percent are acrylic and composed of a polyacrylate matrix, and the remaining 5 percent are composed of specialty polymer matrices, such as phenol-formaldehyde.
Conventional ion exchange resins have a fixed cross-linked polymer matrix with a relatively uniform distribution of mobile ion-active sites throughout the structure. The varying degrees of cross linking in the matrix determine the performance and the “tightness” or “looseness” of the ion exchange resin, since it affects a resin’s properties, such as strength.
Ion exchange resins have either a gel matrix or a microporous matrix and are prepared in granule or spherical (bead) form; the majority of ion exchange resins are spherical.
Key chemical characteristics of ion exchange resins include:
- Capacity: The number of sites available for ion exchange (total capacity) or the measure of the useful performance obtained with the IX resin when it is operating in a column under a prescribed set of conditions (operating capacity).
- Swelling: The hydration of the fixed ionic groups that increases with an increase in capacity to the limits imposed by the resin’s polymer network.
- Selectivity: The affinity of a resin for an ion; selectivity is determined by the ion’s charge and the size of the ion’s hydrated form.
- Kinetics: The speed of the ion exchange.
- Stability: The tendency toward, or rate of, degradation.
Cation, anion, chelating, and adsorbents
Ion exchange resins are identified by the functional group to which they belong. Resins are conventionally classified as:
- Cation exchange resins
- Strong acid (SAC): Characterized by their ability to exchange cations or split neutral salts; they are useful across the entire pH range.
- Weak acid (WAC): Have a high affinity for the hydrogen ion and are therefore easily regenerated with strong acids; they exhibit a high capacity for the alkaline earth metals associated with alkalinity and a more limited capacity for the alkali metals.
- Anion exchange resins
- Strong base (SBA)
- Type 1: The most strongly basic functional group available with the greatest affinity for the weak acids such as silicic acid and carbonic acid that are commonly present during a water demineralization process; the efficiency of regeneration of the resin to the hydroxide form is somewhat lower than Type 2.
- Type 2: Has lower basicity than that of the Type 1 resin, yet it is high enough to remove the weak acidanions for most applications; the chemical stabilityis not as good as that of the Type 1 resins, which are favored for high temperature applications; regeneration efficiency of a Type 2 resin is considerably greater than that of Type 1.
- Weak base (WBA): Contain tertiary amine functionalities, which act as acid adsorbers; capable of sorbing strong acids with a high capacity and are readily regenerated with caustic; particularly effective when used in combination with a strong base anion by providing an overall high operating capacity and regeneration efficiency.
- Strong base (SBA)
- Special chemical groups, such as chelating resins for the hydrometallurgical industry that are particularly applicable for the selective exchange of various heavy metals from alkaline earth and alkali metal solutions.
- Adsorbents: Used for organic compound removal in many industries, such as bioprocessing and applications such as wastewater treatment.
-
What Is Ion Exchange (IX)?
Separating soluble substances by interchanging ions
Whereas solids can be separated from liquid media by means of filtration, soluble substances require different means. Ion exchange is the reversible interchange of ions between a solution with soluble ionized substances and a solid (the ion exchange material, such as a cation resin), in which there is no permanent change in the structure of the solid.
Fundamentally, the ion exchange process is this: A solution passes through or over ion exchange resins, which contain mobile or exchangeable ions. The solute ions that have a stronger affinity to the resins than the ions on the resins are removed from the solution and are replaced by the exchangeable ions on the resins. In water processing, ion exchange typically removes hardness in softening applications and all dissolved ions in demineralization. Ion exchange is also used to remove nitrate, chromate, arsenic, and other specific contaminants from drinking water.
Ion exchange processes can be conducted in continuous or batch mode, with most water treatment IX processes run in a continuous mode. Continuous ion exchange processes take place in a column or vessel containing a deep bed of ion exchange resin beads; water typically flows down through the resin bed. When resin capacity becomes exhausted, the resins are regenerated.
-
Resin Characteristics
Structure and traits
Approximately 85 percent of ion exchange resins in the market are composed of a polystyrene matrix. Another 10 percent are acrylic and composed of a polyacrylate matrix, and the remaining 5 percent are composed of specialty polymer matrices, such as phenol-formaldehyde.
Conventional ion exchange resins have a fixed cross-linked polymer matrix with a relatively uniform distribution of mobile ion-active sites throughout the structure. The varying degrees of cross linking in the matrix determine the performance and the “tightness” or “looseness” of the ion exchange resin, since it affects a resin’s properties, such as strength.
Ion exchange resins have either a gel matrix or a microporous matrix and are prepared in granule or spherical (bead) form; the majority of ion exchange resins are spherical.
Key chemical characteristics of ion exchange resins include:
- Capacity: The number of sites available for ion exchange (total capacity) or the measure of the useful performance obtained with the IX resin when it is operating in a column under a prescribed set of conditions (operating capacity).
- Swelling: The hydration of the fixed ionic groups that increases with an increase in capacity to the limits imposed by the resin’s polymer network.
- Selectivity: The affinity of a resin for an ion; selectivity is determined by the ion’s charge and the size of the ion’s hydrated form.
- Kinetics: The speed of the ion exchange.
- Stability: The tendency toward, or rate of, degradation.
-
IX Resin Types
Cation, anion, chelating, and adsorbents
Ion exchange resins are identified by the functional group to which they belong. Resins are conventionally classified as:
- Cation exchange resins
- Strong acid (SAC): Characterized by their ability to exchange cations or split neutral salts; they are useful across the entire pH range.
- Weak acid (WAC): Have a high affinity for the hydrogen ion and are therefore easily regenerated with strong acids; they exhibit a high capacity for the alkaline earth metals associated with alkalinity and a more limited capacity for the alkali metals.
- Anion exchange resins
- Strong base (SBA)
- Type 1: The most strongly basic functional group available with the greatest affinity for the weak acids such as silicic acid and carbonic acid that are commonly present during a water demineralization process; the efficiency of regeneration of the resin to the hydroxide form is somewhat lower than Type 2.
- Type 2: Has lower basicity than that of the Type 1 resin, yet it is high enough to remove the weak acidanions for most applications; the chemical stabilityis not as good as that of the Type 1 resins, which are favored for high temperature applications; regeneration efficiency of a Type 2 resin is considerably greater than that of Type 1.
- Weak base (WBA): Contain tertiary amine functionalities, which act as acid adsorbers; capable of sorbing strong acids with a high capacity and are readily regenerated with caustic; particularly effective when used in combination with a strong base anion by providing an overall high operating capacity and regeneration efficiency.
- Strong base (SBA)
- Special chemical groups, such as chelating resins for the hydrometallurgical industry that are particularly applicable for the selective exchange of various heavy metals from alkaline earth and alkali metal solutions.
- Adsorbents: Used for organic compound removal in many industries, such as bioprocessing and applications such as wastewater treatment.
- Cation exchange resins
Our approach to ion exchange and ion exchange resins
Since the 1940s, DuPont Water Solutions has developed and introduced into the industry not only stable synthetic organic ion exchange resins, but also products with functionalities that have allowed the companion development of new processes utilizing these ion-exchange-related products. We have pioneered processes such as ion retardation, ion exclusion, acid retardation, desiccation, radium removal, chelation, and catalysis.
Today, we remain dedicated to developing and providing the deepest ion exchange resin portfolio you will find for a wide variety of markets. Our ion exchange resins are part of our Amber Series portfolio.
Find ion exchange resins
View a list of our industry-leading ion exchange resins.
Related industries
Our technologies provide premium solutions for a broad range of industries. Learn more about the industries that depend on ion exchange.

We help offices, schools, hospitals, hotels, and universities enhance their facilities with our water-treatment solutions.

We help everyone’s favorite foods and beverages taste better with advanced separations of sugars, dairy streams, and other nutritional ingredients.

Our technologies and solutions are designed to help you overcome water challenges to produce your desired quantity and quality of industrial utility water.

We develop best-in-class technologies, accompanied by an advanced product portfolio of solutions, to address your crucial wastewater challenges.
We enable the production of some of today’s most popular technologies by facilitating ultrapure water and effective water reuse.

We facilitate the sustainable recovery of valuable raw materials through specialty separation technologies.

We help ensure a steady flow of clean, safe drinking water into local communities with our water-treatment solutions.

We help energy companies improve operational efficiency with specialized water treatment and wastewater reuse.

We enable the smooth and safe operation of power plants through innovative water-treatment and wastewater reuse.

We help improve the safety and quality of drinking water in homes with exceptional water treatment.
Related resources
See what’s possible
Ask us how ion exchange can help you improve the way you remove contaminants from liquids, achieve successful separation, and efficiently catalyze reactions.